ITF Therapeutics was established with an important goal in mind: to build on the legacy of research at Italfarmaco, bring new therapeutic options that may address significant unmet medical needs to people living with rare diseases, and help make these options available for communities in the US.
According to the US National Institutes of Health (NIH), there are more than 7,000 known rare diseases that affect approximately 25 to 30 million people in the United States.1
Currently, there are treatments for only about 600 of these rare diseases.2
ITF Therapeutics and Italfarmaco are focused on changing that number.



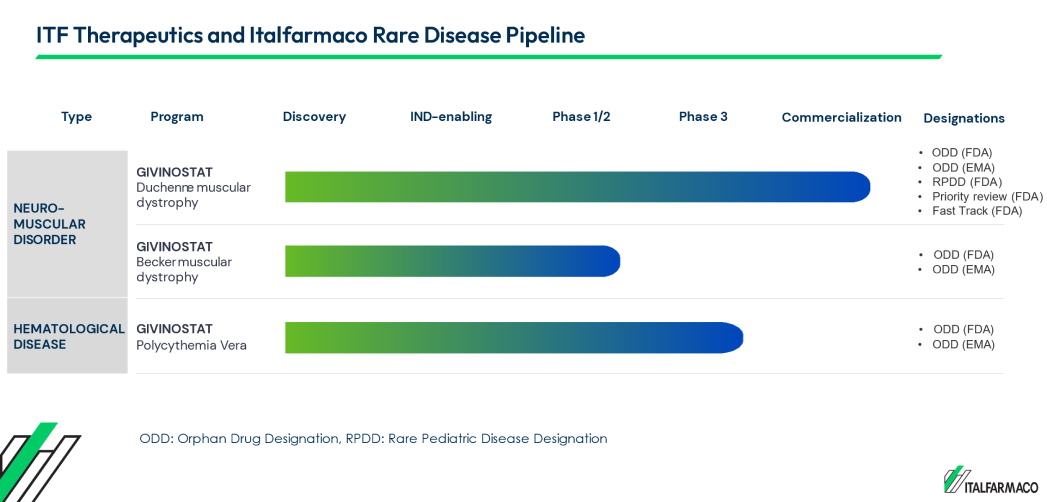
Histone deacetylase (HDAC) inhibitors are a family of proteins that are involved in the post-translational modification of proteins. Our most advanced compound, givinostat (an HDAC inhibitor), has been approved by the FDA for the treatment of DMD in patients six years of age and older.